18A157
A Single Center Experience with the Health Beacon Reporting System: A follow up one year review
Author(s)
Sinead Maguire, Una Martin, Paula Dreelan, Claire Sheehy
Department(s)/Institutions
Department of Rheumatology University Hospital Waterford
Introduction
Medication compliance has been shown to have significant effects on disease control, and quality of life in patients with inflammatory arthritis as well as healthcare costs. Monitoring compliance can be both difficult and labour intensive.
Aims/Background
The AbbVie Care Health Beacon Reporting System (HBRS) monitors the frequency of medication use via subcutaneous pen disposal. Our department had previously noted lower than anticipated compliance rates. This follow up is to examine the change in rates and issues identified with prolonged use.
Method
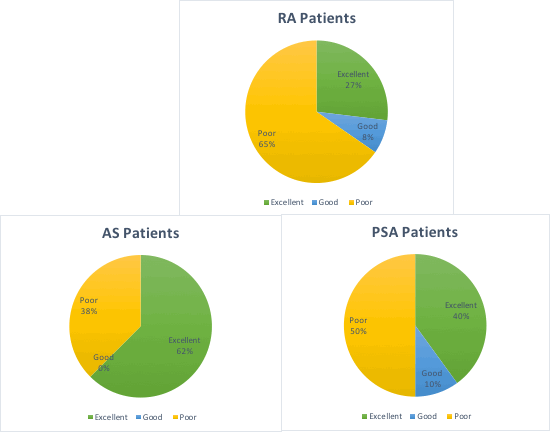
A retrospective review was completed on all UHW patients on Adalimumab currently utilising the HBRS (n=45). Compliance rates were categorised as excellent (81-100%), good (66-80%) or poor (<65%). Patients with a compliance rating of 0% were contacted . This analysis was compared to an earlier analysis done one year ago, shortly after the initiation of the HBRS.
Results
The 45 patients studied had confirmed diagnoses of ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis or juvenile idiopathic arthritis. 56% were categorised as having poor compliance. Further analysis revealed that 40% were registered as 0% compliant. When contacted these 40% confirmed they were not using the HBRS. Reasons given included: change to treatment, poor understanding of device function and improper use of device. Following exclusion of these patients, 74% of patients had good to excellent compliance (table 1). 2017 analysis of HBRS compliance revealed only 40% as good to excellent, however a detailed review of poorly compliant patients was not performed.
Conclusions
This analysis provides a detailed insight into factors affecting the use of the HBRS and true compliance rates. If the data was interpreted blind, the apparent compliance was poor, but when those not using the device were excluded, the numbers were much improved. We would therefore urge caution in interpretation of HBRS results without further exploration. However the HBRS does have potential to be a useful tool to distinguish between poor compliance and medication failure, once use has been confirmed with the patient. Larger patient numbers and increased experience with HBRS will determine how to fully realise its potential.