19A179
A Study of Abatacept Retention in a Tertiary Referral Centre
Author(s)
C Kirby (1), L Corcoran (1), J Eustace (2), S Harney (1)
Department(s)/Institutions
1. Rheumatology Dept., Cork University Hospital 2. Nephrology Dept., Cork University Hospital
Introduction
Abatacept is a T-cell co-stimulation blocker licensed for use in rheumatoid arthritis. It improves symptoms, reduces disease activity and slows radiographic progression.
Aims/Background
We present data on abatacept drug retention in our patients.
Method
We performed a retrospective chart review of all patients treated with abatacept in our hospital since 2010. We gathered data on patient demographics, duration of treatment with abatacept, adverse events and DMARD combinations used with abatacept.
Results
113 patients were treated with abatacept. 93 (82.3%) had rheumatoid arthritis, 14 (12.4%) has a spondyloarthritis, 5 (4.4%) had juvenile idiopathic arthritis and 1 (0.9%) had CPPD-related arthritis.
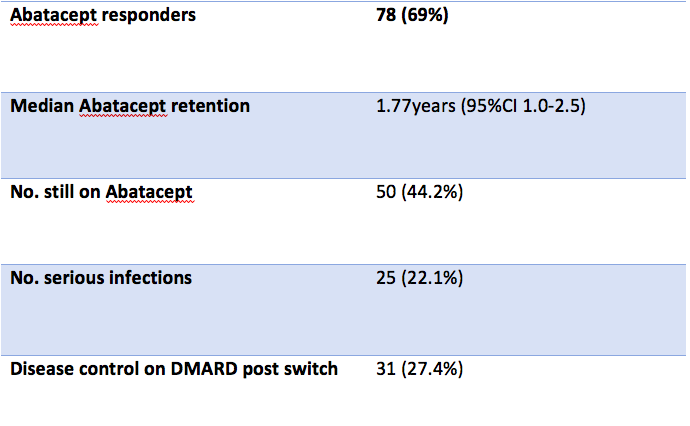
Of 113 patients, 50 (44.2%) remain on treatment. Of those who had treatment discontinued, 23 (20.4%) had primary failure, 16 (14.2%) had secondary failure and 18 (15.9%) had treatment discontinued due to adverse events.
Of DMARDs used prior to abatacept, 73 (64.6%) patients had methotrexate (MTX), 30 (26.5%) had leflunomide (LEF), 30 (26.5%) had sulfasalazine (SLZ), and 81 (71.7%) had a prior tumour necrosis factor inhibitors (TNFi).
49 patients (43.4%) were co-prescribed DMARDs in combination with abatacept as follows: glucocorticoids in 15 (13.3%) patients, MTX in 38 (33.6%), LEF in 11 (9.7%), Hydroxychloroquine in 7 (6.2%) and SLZ in 6 (5.3%).
Serious infections were observed in 25 (22.1%) patients, cardiovascular events in 1 (0.9%) patient, injection site reactions in 1 (<1%) patient and other mucocutaneous adverse events in 12 (10.6%).
After abatacept discontinuation, 14 patients (12.4%) were switched to a synthetic DMARD, 14 (12.4%) were switched to a TNFi, 11 (9.7%) were switched to an IL-6 inhibitor, 8 (7.1%) were switched to a janus kinase inhibitor (JAKi), and 10 (8.6%) were switched to other biologic DMARDs. 31 (27.4%) achieved disease control on their new DMARD. Data on disease control post switch was unavailable in 43 (38.1%) patients.
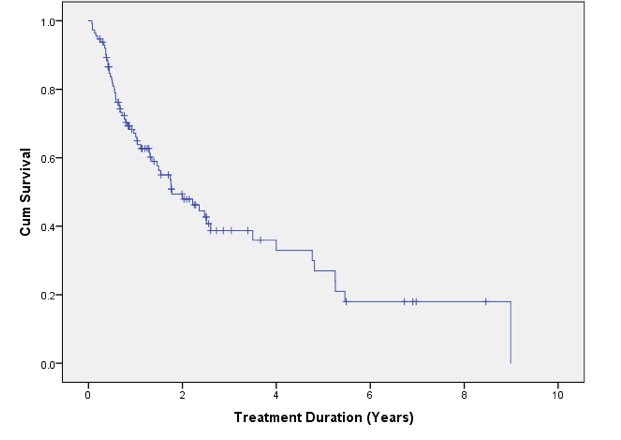
Abatacept achieved a clinical response in 78 (69%) patients. Median retention of abatacept in years was 1.77 (95% CI 1.0-2.5).
Conclusions
Nearly half of patients commenced on abatacept in our center remain on the drug and nearly 2/3 of all patients treated achieved a meaningful clinical response. Median retention of abatacept was 1.77 years.