19A118
Efficacy and Safety of Filgotinib for Patients with Rheumatoid Arthritis Naïve to Methotrexate Therapy: FINCH 3 Primary Outcome Results
Author(s)
Oliver Fitzgerald (1), Rene Westhovens (2), William F.C. Rigby (3), Désirée van der Heijde (4), Daniel W.T. Ching (5), Beatrix Bartok (6), Franziska Matzkies (6), Zhaoyu Yin (6), Ying Guo (6), Chantal Tasset (7), John S. Sundy (6), Neelufar Mozaffarian (6), Osvaldo Daniel Messina (8), Robert B.M. Landewé (9), Tatsuya Atsumi (10), Gerd R. Burmester (11)
Department(s)/Institutions
1. University College Dublin, Dublin, Ireland; 2. University Hospitals Leuven, Leuven, Belgium; 3. Dartmouth College, Lebanon, NH, USA; 4. Leiden University Medical Center, Leiden, Netherlands; 5. Timaru Hospital, Timaru, New Zealand; 6. Gilead Sciences, Inc., Foster City, CA, USA; 7. Galapagos NV, Mechelen, Belgium; 8. Cosme Argerich Hospital and IRO Medical Center, Buenos Aires, Argentina; 9. Amsterdam University Medical Center, Amsterdam, Netherlands; 10. Hokkaido University, Sapporo, Japan; 11. Charité – University Medicine Berlin, Berlin, Germany
Introduction
Filgotinib (FIL), an oral selective Janus kinase 1 inhibitor, has shown efficacy and was well tolerated for rheumatoid arthritis (RA) treatment.
Aims/Background
To compare efficacy and safety of FIL with and without methotrexate (MTX) in patients with RA naïve to MTX.
Method
This Phase 3, double-blind, active-controlled study randomised patients with moderately to severely active RA (2:1:1:2) to FIL 200mg daily + MTX weekly (up to 20mg), FIL 100mg + MTX, FIL 200mg (+placebo [PBO]), or MTX (+PBO) up to 52 weeks; results are through Week 24. Primary endpoint was proportion achieving ACR20 response at Week 24; additional assessments included ACR50/70 responses; DAS28-CRP ≤3.2 and <2.6, and changes in van der Heijde mTSS, and patient-reported outcomes (PROs). Safety endpoints included adverse event types and rates. Logistic regression adjusting for stratification factors with non-responder imputation was used for treatment comparisons for binary endpoints. Mixed-effect model adjusting for baseline value, stratification factors, treatment, visit and treatment by visit interaction was used for continuous endpoints.
Results
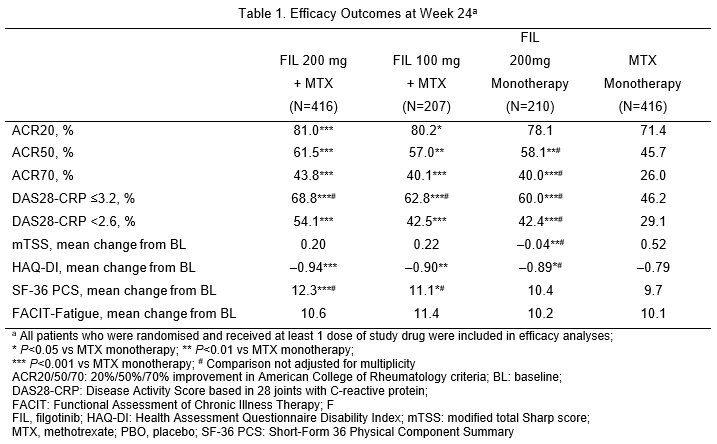
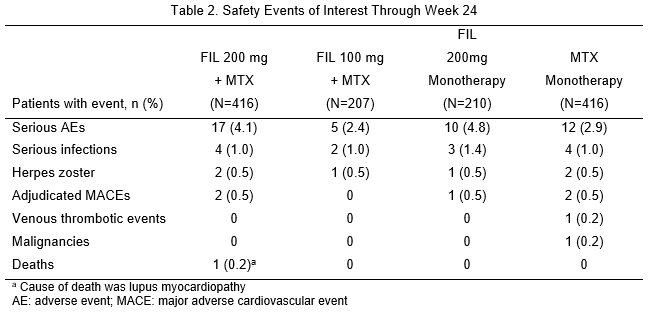
1,249 patients received the study drug (416 FIL 200mg+MTX; 207 FIL 100mg+MTX; 210 FIL 200mg monotherapy; 416 MTX monotherapy). 76.9% were female; mean time since RA diagnosis 2.2 years (median 0.4 years); mean (standard deviation) DAS28-CRP 5.7 (1.0); and 35.9% using oral steroids at baseline. At Week 24, significantly more patients on FIL 200mg + MTX (81.0%; P<0.001) and FIL 100mg + MTX (80.2%; P<0.05) achieved ACR20 response compared with MTX monotherapy (71.4%; Table 1). Compared with MTX monotherapy, more patients receiving FIL with or without MTX achieved ACR50 and ACR70, DAS28-CRP <2.6 and ≤3.2 and SF-36 PCS improvements (Table 1). Onset of activity was rapid, with significantly more achieving ACR50 and DAS28-CRP <2.6 with FIL than MTX at Week 2. FIL safety was consistent with prior studies through Week 24 (Table 2).
Conclusions
FIL + MTX had significant improvements in RA signs and symptoms, physical function and PROs compared to MTX alone and was well tolerated in patients with early active RA naïve to MTX. Clinically meaningful response to FIL occurred as early as 2 weeks after treatment initiation.