18A166
Genetics of Rare Disease: A novel familial RELA truncation associates with Behçet’s-like Mucocutaneous Ulceration Syndrome
Author(s)
Emma Dorris, Fahd Adeeb, Dylan Lawless, Eoin Cummins, Sinisa Savic, Sandy Fraser, Anthony G. Wilson
Department(s)/Institutions
UCD Centre for Arthritis Research University of Limerick The University of Leeds UCD School of Medicine Croom Orthopedic Hospital
Introduction
Bechet’s disease (BD) is a heterogeneous multifactorial auto-inflammatory condition characterized by recurrent episodes of oral and genital ulceration, uveitis and skin lesions, with less frequent involvement of the gastrointestinal tract, large blood vessels and central nervous system. The NF-κB pathway is a ‘master-regulator’ of immune and inflammatory signaling, with the ability to control the expression of key inflammatory genes and genes associated with apoptosis and proliferation.
Aims/Background
The aim of this study is to identify potentially causative mutations. The objective was to determine genotype-phenotype association with the aim of earlier and better disease suppression, preventing tissue damage and improving the quality of life of affected children.
Method
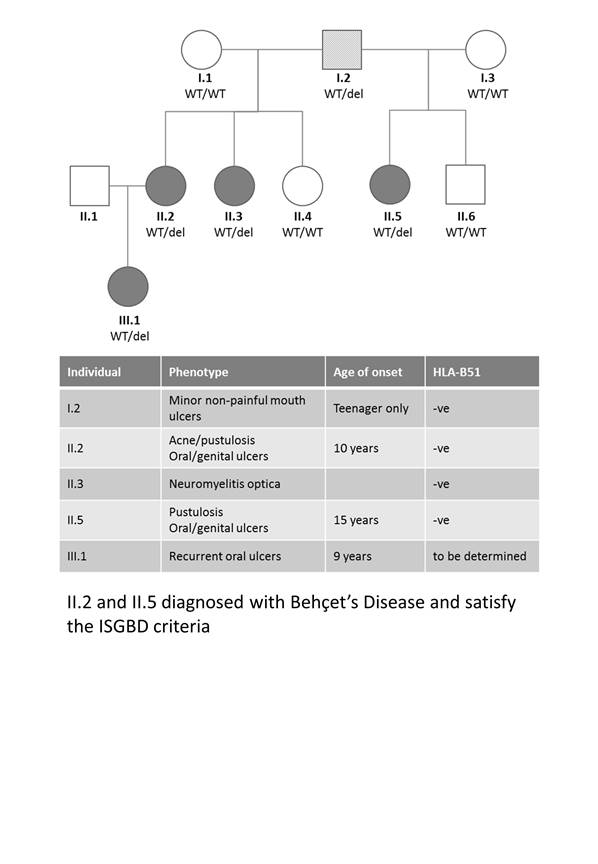
This study involved a 3-generation family with Behçet’s-like mucocutaneous ulceration syndrome; primarily involving childhood-onset chronic oral and genital ulcers (figure 1). ISGBD criteria were used to diagnose Behçet’s Disease (BD). DNA was isolated from PBMCs from affected patients and non-affected familial controls. DNA sequencing identified a cysteine deletion at position 1459 in RELA which segregated with the condition. Immunoblot analysis of RELA confirmed protein truncation. PBMCs were stimulated with TNF or IL1B and NFkB phosphorylation, apoptosis markers, translocation and transcriptional activation was measured relative to unstimulated controls.
Results
A heterozygous cysteine deletion at position 1459 in RELA was detected in affected individuals. This mutation is coding, inducing a frameshift His487ThrfsTer7, predicted to produce a truncated protein of 492 amino acids which would result in a ~6kDa smaller protein. This truncation was confirmed by immunoblot. Preliminary data indicates RelAHis487ThrfsTer7 heterozygotes have altered kinetics in inflammatory and apoptosis pathways in response to inflammatory stimulants.
Conclusions
This study gives novel information on both the genetic basis and biological mechanisms of inherited BD. Crucially, the His487ThrfsTer7 mutation interrupts the two C-terminal RELA transactivating domains. Our study supports several recently published studies that loss-of-function mutations in the NF-κB pathway are linked with the development of familial early-onset BD-like syndromes. Understanding both the genetic basis and biological mechanisms facilitates personalized medicines approaches that target the primary disease mediators, which result in earlier disease control and reduced tissue damage.